Catalytic study of highly recoverable chitosan supported Cu(II) Schiff base complex for alkanes oxidation

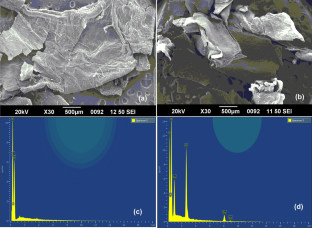
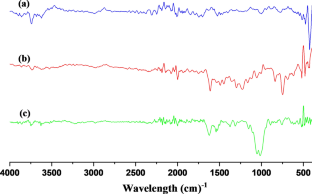
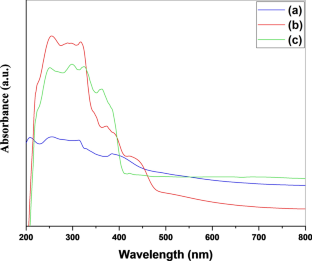
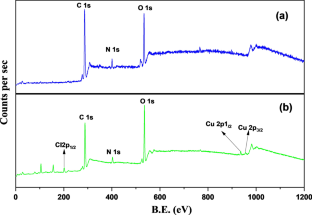
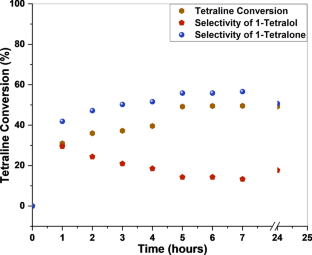
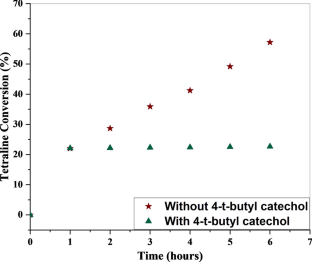
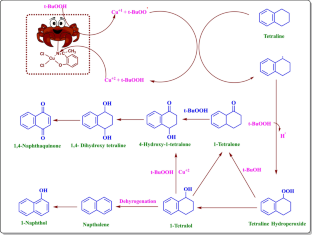
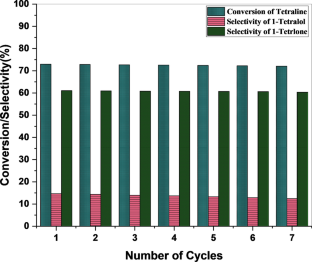
We have synthesized a chitosan anchored Cu(II) Schiff base heterogeneous catalyst. Schiff base ligand was prepared by condensation of chitosan (Cs) with ortho-hydroxyl acetophenone (OHACP).It is abbreviated as [Cs-OHACP]. Then Cu(II) heterogeneous catalyst2> was prepared by the reaction of [Cs-OHACP] and cupric chloride. The synthesized catalyst, 2> was characterized byscanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX), Brunauer–Emmett–Teller (BET) surface area, X-ray powder diffraction (XRD), thermogravimetric analysis (TGA), Fourier transform infrared (FTIR), Ultraviolet visible near-infrared (UV–VIS–NIR), X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) studies. The catalytic performance of heterogeneous catalyst was tested for alkane oxidation using 70% tert-butylhydroperoxide (TBHP) as an oxidant. The influencing factors that affect the rate of reaction such as various oxidants, solvents, reaction time, molar ratio of tetralin to oxidant, catalyst amount and reaction temperature were investigated for optimization of alkane oxidation. The heterogeneous catalyst 2> is easy to separate, handle and recover. It was recycled seven times.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
Instant access to the full article PDF.
Rent this article via DeepDyve
Similar content being viewed by others

Synthesis and DFT studies of robust heterogeneous Co(III) catalyst for liquid phase oxidation of tetralin
Article 01 April 2024
Epoxidation of alkenes with NaIO4 catalyzed by an efficient and reusable natural polymer-supported ruthenium(III) salophen catalyst
Article Open access 17 November 2015
Copper(II)-Ethanolamine Triazine Complex on Chitosan-Functionalized Nanomaghemite for Catalytic Aerobic Oxidation of Benzylic Alcohols
Article 02 July 2020
Explore related subjects
Data availability
References
- Debono N, Iglesias M, Sanchez F (2007) New pyridine ONN-pincer gold and palladium complexes: synthesis, characterization and catalysis in hydrogenation, hydrosilylation and C–C cross-coupling reactions. Adv Synth Cata 349:2470–2476 ArticleCASGoogle Scholar
- Nomura N, Ishii R, Akakura M, Aoi K (2002) Stereoselective ring-opening polymerization of racemic lactide using aluminum-achiral ligand complexes: exploration of a chain-end control mechanism. Am Chem Soc 124:5938–5939 ArticleCASGoogle Scholar
- Miller JA, Jin W, Nguyen ST (2002) An efficient and highly enantio- and diastereoselective cyclopropanation of olefins catalyzed by schiff-base ruthenium(II) complexes. Angew Chem 114:3077–3080 ArticleGoogle Scholar
- Abu-Dief AM, Mohamed IMA (2015) A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ J Basic App Sci 4(2):119–133 Google Scholar
- Dhakshinamoorthy A, Alvaro M, Garcia H (2011) Metal–organic frameworks as heterogeneous catalysts for oxidation reactions. Catal Sci Technol 1:856–867 ArticleCASGoogle Scholar
- Khare S, Kirar JS, Parashar S (2019) Solvent-free oxidation of ethylbenzene over LDH-hosted Co(II) Schiff base of 2-hydroxy-1- naphthaldehyde and 4-amino benzoic acid. Inorg Nano-Met Chem 49:204–216 ArticleCASGoogle Scholar
- Al Zoubi W, Ko YG (2016) Organometallic complexes of Schiff bases: Recent progress in oxidation catalysis. J Organomet Chem 822:173–188 ArticleCASGoogle Scholar
- Khare S, Chokhare R, Shrivastava P, Kirar JS, Parashar (2017) α-Zirconium phosphate supported metal–salen complex: synthesis, characterization and catalytic activity for cyclohexane oxidation. J Porous Mater 24:855–866 ArticleCASGoogle Scholar
- Frank HG, Stadelhofer JW (1988) Industrial Aromatic Chemistry. Springer-Verlag, Berlin BookGoogle Scholar
- Mizukami F, Imamura J (1979) US 4 175 098
- Groggins PH, Nagel RH (1934) Studies in the Friedel and Crafts reaction preparation of ketones and keto acids. Ind Eng Chem 26:1313–1316 ArticleCASGoogle Scholar
- Feldberg L, Sasson Y (1996) Copper catalyzed oxidation of tetralin to 1-(tert-butylperoxy)-tetralin by aqueous tert-butylhydroperoxide under phase transfer conditions. Tetrahedron Lett 37:2063–2066 ArticleCASGoogle Scholar
- Navarro M, Escobar A, Landaeta VR, Visbal G, Lopez-Linares F, Luis ML, Fuentes A (2009) Catalytic oxidation of tetralin by biologically active copper and palladium complexes. Appl Catal A-Gen 363:27–31 ArticleCASGoogle Scholar
- Kharat AN, Jahromi BT, Bakhoda A (2012) Manganese(II), cobalts (II) and nickel(II) complexes of tris(2-pyridyl) phosphine and their catalytic activity toward oxidation of tetralin. Transition Met Chem 37:63–69 ArticleCASGoogle Scholar
- Khare S, Shrivastava P (2016) liquid phase solvent-less cyclohexane oxidation catalyzed by covalently anchored transition-metal schiff base complexon a-titanium phosphate. Catal Lett 146:319–332 ArticleCASGoogle Scholar
- Sheldon RA, Kochi JK (1981) Metal-catalyzed oxidation of organic compounds. Academic, New York, p 34 Google Scholar
- Guibal E (2005) Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci 30:71–109 ArticleCASGoogle Scholar
- Kirar JS, Khare S, Tiwari N (2021) Fabrication and characterization of Cu nanoparticles dispersed on Zn Al-layered double hydroxide nanocatalysts for the oxidation of cyclohexane. Chem Sel 6:11557–11568 CASGoogle Scholar
- Chakraborty T, Chakraborty A, Maity S, Das D, Chattopadhyay T (2019) Conglomerated system of Ag nanoparticles decorated Al2O3 supported cobalt and copper complexes with enhanced catalytic activity for oxidation reactions. Mol Catal 462:104–113 ArticleCASGoogle Scholar
- Ispir E (2014) Synthesis and characterization of silica–supported Schiff base ligands and their metal complexes: applications as catalysts for the oxidation of alkanes. Phosphorus Sulfur Silicon 189:1644–1655 ArticleCASGoogle Scholar
- Kumari S, Ray S (2020) Zeolite encapsulated Ni(II) Schiff-base complexes: improved catalysis and site isolation. New J Chem 44:14953–14963 ArticleCASGoogle Scholar
- Antony R, Arun T, Theodore David Manickam S (2019) A review on applications of chitosan-based Schiff bases. Int J Biol Macromo 129:615–633 ArticleCASGoogle Scholar
- Lee M, Den CB, W, (2015) Chitosan as a natural polymer for heterogeneous catalysts support: a short review on its applications. Appl Sci 5:1272–1283 ArticleCASGoogle Scholar
- Baran T, Menteş A (2016) Polymeric material prepared from Schiff base based on O-carboxymethyl chitosan and its Cu(II) and Pd(II) complexes. J Mol Struct 1115:220–227 ArticleCASGoogle Scholar
- Kramareva NV, Stakheev AY, Tkachenko OP, Klementiev KV, Grunert W, Finashina ED, Kustov LM (2004) Heterogenized palladium chitosan complexes as potentialcatalysts in oxidation reactions: study of the structure. J Mol Catal 209:97 ArticleCASGoogle Scholar
- Li-xia W, Zi-wei W, Guo-song W, Xiao-dong L, Jian-guo R (2010) Catalytic performance of Chitosan-Schiff basesupported Pd/Co bimetallic catalyst for acrylamide with phenyl halide. Polym Adv Technol 21:244 ArticleGoogle Scholar
- Reddy KR, Rajgopal K, Maheshwari CU, Katam ML (2006) Chitosan hydrogel: a green and recyclable biopolymer catalyst for aldol and Knoevenagel reactions. New J Chem 30:1549–1552 ArticleCASGoogle Scholar
- Demetgul C, Serin S (2008) Synthesis and characterization of a new vic-dioxime derivative of chitosan and its transition metal complexes. Carbohydr Polym 72:506–512 ArticleGoogle Scholar
- Chang Y, Wang Y, Zha F, Wang R (2004) Preparation and catalytic properties of chitosan bound Schiff base copper complexes. Polym Adv Technol 15:284–286 ArticleCASGoogle Scholar
- Tong J, Li Z, Xia C (2005) Highly efficient catalysts of Chitosan-Schiff base Co(II) and Pd(II) complexes for aerobic oxidation of cyclohexane in the absence of reductants and solvents. J Mol Catal 231:197–203 ArticleCASGoogle Scholar
- Xue L, Zhou DJ, Tang L (2004) The asymmetric hydration of 1-octene to (S)-(+)-2-octanol with a biopolymer–metal complex, silica-supported chitosan–cobalt complex. React Funct Polym 58:117–121 ArticleCASGoogle Scholar
- Hu DD, Cui YL, Dong XL (2001) Studies on Co-Salen immobilized onto N-(4-pyridylmethylidene)–chitosan. React Funct Polym 48:201–207 ArticleCASGoogle Scholar
- Baran T, Açıksöz E, Menteş A (2015) Carboxymethyl chitosan Schiff base supported heterogeneous palladium(II) catalysts for Suzuki cross-coupling reaction. J Mol Catal A Chem 407:47–52 ArticleCASGoogle Scholar
- Sun W, Xia CG, Wang HW (2002) Efficient heterogeneous catalysts for the cyclopropanation of olefins. New J Chem 226:755–758 ArticleGoogle Scholar
- Baran T (2019) Highly recoverable, reusable, cost-effective, and Schiff base functionalized pectin supported Pd(II) catalyst for microwave-accelerated Suzuki cross-coupling reactions. Int J Biol Macromol 127:232–239 ArticleCASPubMedGoogle Scholar
- Shafiei N, Nasrollahzadeh M, Baran T, Baran NY (2021) Pd nanoparticles loaded on modified chitosan-Unye bentonite microcapsules: a reusable nanocatalyst for Sonogashira coupling reaction. Carbohy Polym 262:117920 ArticleCASGoogle Scholar
- Hamed AA, Abdelhamid IA, Saad GR, Elkady NA, Elsabee MZ (2020) Synthesis, characterization and antimicrobial activity of a novel chitosan Schiff bases based on heterocyclic moieties. Int J Biol Macromol 153:492–501 ArticleCASPubMedGoogle Scholar
- Kamiya Y, Ingold K (1964) The Metal-catalyzed autoxidation of Tetralin: III. catalysis by manganese, copper, nickel, and iron. Can J Chem 42:1027–1043 ArticleCASGoogle Scholar
- Mizukami F, Imamura J (1978) Liquid-phase oxidation of hydrocarbons with molecular oxygen. I. the effects of metal ions and their ligands on product distribution in the oxidation of tetralin in acetic acid. Bull Chem Soc Jpn 51:1404–1412 ArticleCASGoogle Scholar
- Frank HG, Stadelhofer JW (1988) Industrial Aromatic Chemistry. Springer-Verlag, Heidelberg, p 313 BookGoogle Scholar
- Dhakshinamoorthy A, Alvaro M, Garcia H (2009) Metal organic frameworks as efficient heterogeneous catalysts for the oxidation of benzylic compounds with t-butylhydroperoxide. J Cat 267(1):1–4 ArticleCASGoogle Scholar
- Bhattacharjee S, Lee YR, Ahn WS (2017) Oxidation of tetraline to 1-tetralone over CrAPO-5. Korean J Chem Eng 34(3):701–705 ArticleCASGoogle Scholar
- Bhattacharjee S, Jeong KE, Jeong SY, Ahn WS (2010) Synthesis of a sulfonato-salen-nickel (II) complex immobilized in LDH for tetralin oxidation. New J Chem 34:156–162 ArticleCASGoogle Scholar
- Kim J, Bhattacharjee S, Jeong KE, Jeong SY, Ahn WS (2009) Selective oxidation of tetraline over a chromium terephthalate metal organic framework, MIL-101. Chem Commun 26:3904–3906 ArticleGoogle Scholar
- Neeli CKP, Kannapu HPR, Kalevaru VN, Kamaraju SRR, Burri DR (2018) An efficient and selective benzylic oxidation of tetralin to 1-tetralone on Cu (II) immobilized γ-Fe2O3@SBA-15 magnetic Nano catalyst in green water medium without base or additives. Mol Catal 45:74–84 ArticleGoogle Scholar
- Wan C, Zhu M, Du L, Xu L, Ye M, An Y (2019) Highly efficient aerobic oxidation of tetralin to α-tetralone over MnOx–CoOy/γ-Al2O3 catalysts. Catal Commun 125:87–92 ArticleCASGoogle Scholar
- LlabrésIXamena FX, Casanova O, Galiasso Tailleur R, Garcia H, Corma A (2008) Metal organic frameworks (MOFs) as catalysts: a combination of Cu2+ and Co2+ MOFs as an efficient catalyst for tetralin oxidation. J Catal 255:220–227 ArticleGoogle Scholar
- Louis B, Detoni C, Carvalho NMF, Duarte CD, Antunes OAC (2009) Cu(II) bipyridine and phenantroline complexes: Tailor-made catalysts for the selective oxidation of tetralin. Appl Catal A: Gen 360:218–225 ArticleCASGoogle Scholar
- Ma Y, Zeng M, He J, Duana L, Wang J, Li J, Wang J (2011) Syntheses and characterizations of cobalt doped mesoporous alumina prepared using natural rubber latex as template and its catalytic oxidation of tetralin to tetralone. Appl Catal A: Gen 396:123–128 ArticleCASGoogle Scholar
- Shaikh RA, Chandrasekar G, Biswas K, Choi JS, Son WJ, Jeong SY, Ahn WS (2008) Tetralin oxidation over chromium-containing molecular sieve catalysts. Catal Today 132:52–57 ArticleCASGoogle Scholar
- Razi R, Abedini M, Kharat AN, Amini MM (2008) Oxidation of tetralin with molecular oxygen by vanadium-substituted polyoxometalate. Catal Commun 9:245–249 ArticleCASGoogle Scholar
- Parashar S, Khare S (2019) Transition metal Schiff base complexes supported on layered double hydroxide: synthesis, characterization and catalytic activity for the oxidation of toluene. Reac Kinet Mech Cat 127:469–488 ArticleCASGoogle Scholar
- Khare S, Shrivastava P, Chokhare R, Kirar JS, Parashar S (2018) Catalytic liquid phase oxidation of cyclohexane with tert-butylhydroperoxide over transition metal exchanged α-zirconium phosphate. Indian J Chem Sect A 57:427–443 Google Scholar
- Kirar JS, Khare S, Tiwari N (2021) Transition metal Schiff base complexes supported on layered double hydroxide: synthesis, characterization and catalytic activity for the oxidation of toluene. Reac Kinet Mech Cat 132:1025–1046 ArticleCASGoogle Scholar
- Parashar S, Khare S (2019) Synthesis, characterization and catalytic activity of Cu(II) Schiff base complexes intercalated in layered double hydroxide for the oxidation of styrene. Indian J Chem 58A:753–762 CASGoogle Scholar
- Sagunthala Devi PR, Theodore David S, Biju Bennie R, Joel C, Daniel Abraham S (2023) Investigation on the effect of electron beam impact on chitosan anchored mixed ligand Schif base complexes for cyclohexane oxidation. Reac Kinet Mech Cat 136:963–979 ArticleGoogle Scholar
- Inukai Y, Chinen T, Matsuda T, Kaida Y, Yasuda S (1998) Selective separation of germanium(IV) by 2,3-dihydroxypropyl chitosan resin. J Analytica Chimica Acta 371:187–193 ArticleCASGoogle Scholar
- El-Atawy MA, Khalil KD, Bashal AH (2022) Chitosan capped copper oxide nanocomposite: efficient, recyclable, heterogeneous base catalyst for synthesis of nitroolefins. Catalysts 12:964 ArticleCASGoogle Scholar
- Khan A, Toufiq AM, Tariq F, Khan Y, Hussain R, Akhtar N, Rahman S (2019) Influence of Fe doping on the structural, optical and thermal properties of α-MnO2 nanowires. Mater Res Express 6:6 Google Scholar
- Antony R, Manickam STD, Saravanan K, Karuppasamy K, Balakumar S (2013) Synthesis, spectroscopic and catalytic studies of Cu(II), Co(II) and Ni(II) complexes immobilized on Schiff base modified chitosan. J Mol Stru 1050:53–60 ArticleCASGoogle Scholar
- Nakamoto K (1986) Coordination compounds, Infrared and Raman spectra of inorganic and coordination compounds, 4th edn. Wiley, NewYork Google Scholar
- Baran T, Mentes A, Arslan H (2015) Synthesis and characterization of water soluble O-carboxymethyl chitosan Schiff bases and Cu(II) complexes. Int J Bio Macromol 72:94–103 ArticleCASGoogle Scholar
- Khare S, Chokhare R (2012) Oxidation of cyclohexene catalyzed by Cu(Salen) intercalated α-zirconium phosphate using dry tert-butylhydroperoxide. J Mol Catal A Chem 353–354:138–147 ArticleGoogle Scholar
- Arshad F, Munir A, Kashif QQ, ulHaq T, Iqbal J, Sher F, Hussain I (2020) Controlled development of higher-dimensional nanostructured copper oxide thin films as binder free electrocatalysts for oxygen evolution reaction. Int J hydrog energy 45:16583–16590 ArticleCASGoogle Scholar
- Mondal P, Sinha A, Salam N, Roy AS, Jana NR, Islam SM (2013) Enhanced catalytic performance by copper nanoparticle–graphene based composite. RSC Adv 3:5615–5623 ArticleCASGoogle Scholar
- Moulder JF., Stickle WF., Peter E. Sob (BookZZ.org)
- Frisch, MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JJE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09 Revision B.01 Gaussian Inc Wallingford CT
- Abdel-Rahman LH, Ismail NM, Ismael M, Abu-Dief AM, Abdel- Hameed Ahmed E (2017) Synthesis, characterization, DFT calculations and biological studies of Mn(II), Fe(II), Co(II) and Cd(II) complexes based on a tetradentate ONNO donor Schiff base ligand. J Mol Struct 1134:851–862 ArticleCASGoogle Scholar
- Shimizu I, Morimoto Y, Faltermeier D, Kerscher M, Paria S, Abe T, Sugimoto H, Fujieda N, Asano K, Suzuki T, Comba P, Itoh S (2017) Tetrahedral copper(II) complexes with a labile coordination site supported by a tris-tetramethylguanidinato, Ligand. Inorg Chem 56:9634–9645 ArticleCASPubMedGoogle Scholar
- Mathammal R, Sangeetha K, Sangeetha M, Mekala R, Gadheeja S (2016) Molecular structure, vibrational, UV, NMR, HOMO-LUMO, MEP, NLO, NBO analysisof 3, 5 di tert butyl 4 Hydroxy benzoic acid. J Mol Struct 1120:1–14 ArticleCASGoogle Scholar
- Basha MT, Alghanmi RM, Shehata MR, Abdel-Rahman LH (2019) Synthesis, structural characterization, DFT calculations, biological investigation, molecular docking and DNA binding of Co(II), Ni(II) and Cu(II) nanosized Schiff base complexes bearing pyrimidine moiety. J Mol Struct 1183:298–312 ArticleCASGoogle Scholar
- Hossain MS, Khushy KA, Latif MA, Hossen MF, Asraf MA, Zahan MKE, Abdou A (2022) Co (II), Ni (II), and Cu (II) complexes containing isatin-based Schiff Base ligand: synthesis, physicochemical characterization, DFT calculations, antibacterial Activity, and molecular docking analysis. Russ J Gen Chem 92:2723–2733 ArticleCASGoogle Scholar
- Yousef TA (2020) Structural, optical, morphology characterization and DFT studies of nano sized Cu(II) complexes containing schiff base using green synthesis. J Mol Struct 1215:128180 ArticleCASGoogle Scholar
- Abdou A, Mawgoud MA (2022) Synthesis, structural elucidation, and density functional theory investigation of new mononuclear Fe (III), Ni (II), and Cu (II) mixed-ligand complexes: biological and catalase mimicking activity exploration. Appl Organomet Chem 36:6600 ArticleGoogle Scholar
- Elkanzi NAA, Hrichi H, Salah H, Albqmi MA, Abdou A (2023) Synthesis, physicochemical properties, biological, molecular docking and DFT investigation of Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) complexes of the 4-[(5-oxo-4,5-dihydro-1,3-thiazol-2-yl)hydrazono]methyl>phenyl 4-methylbenzenesulfonate Schiff-base ligand. Polyhedron 230:116219 ArticleCASGoogle Scholar
- Abdou A, Mostafa HM, Abdel-Mawgoud AM (2022) Seven metal-based bi-dentate NO azocoumarine complexes: synthesis, physicochemical properties, DFT calculations, drug-likeness, in vitro antimicrobial screening and molecular docking analysis. Inorg Chim Acta 539:121043 ArticleCASGoogle Scholar
- Abdou A, Mostafa HM, Abdel-Mawgoud MA, Sohag (2022) Molecular modeling, breast cancer, and hepatitis a, b, c molecular docking investigation of (2E)-1-phenyl-butane-1, 2, 3-trione 2-[(2-oxo-2H-chromene-6-yl) hydrazone. Sohag J Sci 7:167–173 Google Scholar
- Elkanzi NAA, Ali AM, Albqmi M, Abdou A (2022) New Benzimidazole-based Fe (III) and Cr (III) complexes: characterization, bioactivity screening, and theoretical implementations using DFT and molecular docking analysis. Appl Organomet Chem 36:6868 ArticleGoogle Scholar
- Shukla N, Gaur P, Raidas ML, Chaurasia B (2020) Tailored synthesis of unsymmetrical tetradentate ONNO schiff base complexes of Fe(IIl), Co(II) and Ni(II): Spectroscopic characterization, DFT optimization, oxygen-binding study, antibacterial and anticorrosion activity. J Mol Struct 1202:127362 ArticleCASGoogle Scholar
Acknowledgements
We are very thankful to Head, School of Chemical Sciences, DAVV, Indore. UGC-DAE Consortium of Scientific Research, D AVV, Indore for providing analytical facilities such as XRD and XPS. STIC Cochin for SEM and EDX facilities, IIT Madras for DRUV-Vis facility, Material analysis and research centre (MARC) Bangalore for BET surface area and IISER Bhopal for GC-MS facility.
Author information
Authors and Affiliations
- School of Chemical Sciences, Devi Ahilya Vishwavidyalaya, Takshashila Campus, Khandwa Road, Indore, MP, 452001, India Savita Khare & Neha Tiwari













