 Solid works model" />
Solid works model" />American Cleanroom Systems® are experts in cleanroom design and have extensive experience designing, manufacturing, and installing modular cleanrooms for leading pharmaceutical, medical device and industrial companies.
 Solid works model" />
Solid works model" />
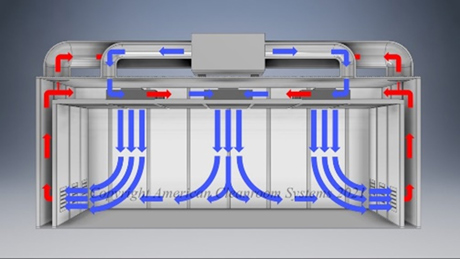
Low velocity vertical laminar air flows to maximize cleanroom effectiveness and efficiency.
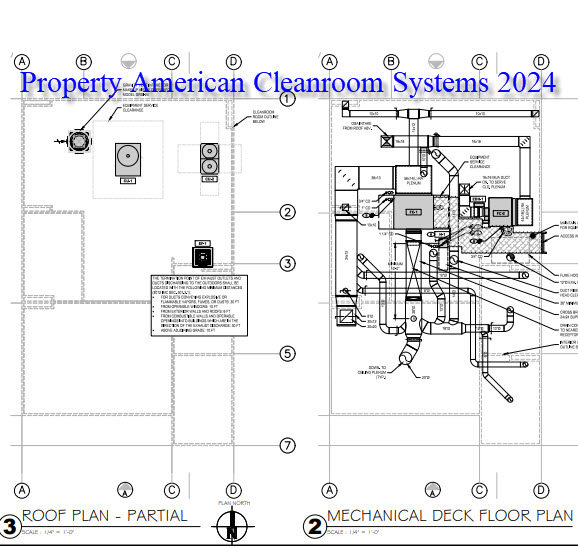
MEP design including heat load, exhaust cfm, make up air, hardware specification and system design.
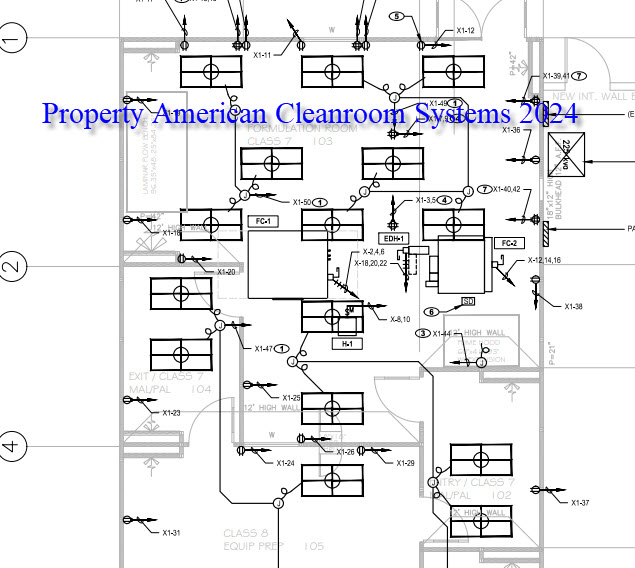
Electrical design including lighting, electrical outlets, panels and wiring diagrams.

Uniform temperature and humidity control and distribution for maintaining cleanroom environment specifications.

Floor to ceiling clear viewing panels provide improved worker and manufacturing visibility.

Custom environmental systems for tight temperature and humidity requirements.
American Cleanroom Systems® has extensive experience designing and engineering custom cleanrooms to unique and strict requirements for pharmaceutical, medical device and industrial cleanroom clients. Our design team creates and provides complete design specifications for each project.

Electronics Modular Cleanroom

Manufacturing Modular Cleanroom

Modular Cleanroom

Chiller for Research & Development Modular Cleanroom
Modular Cleanroom
SQFT: 2000 SF
Cleanroom Class: Class 100 / ISO-5
Specific Requirements:
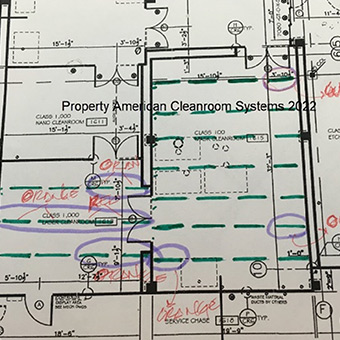

We have extensive experience working with end-users, general contractors, architects and engineering firms.
A:
A: Pharmaceutical manufacturers are subject to FDA validation of their manufacturing which typically specify use of a clean room to ensure the quality of the manufactured pharmaceutical product. Sterility is highest priority. Pharmaceutical cleanrooms focus on both non viable (inanimate) and viable (live) contamination. They typically use laser particle counters to measure non viable contamination levels and settling plates with culture media to measure viable contamination levels. Pharmaceutical cleanrooms use aggressive chemical and UV light cleaning techniques to maintain sterility.
A: Cleanrooms are used in any industry that wants to control contamination in their facility. It is common to see pharmaceutical cleanrooms, medical device cleanrooms, semiconductor cleanrooms, electronic cleanrooms, aerospace cleanrooms, food cleanrooms, USP797 compounding pharmacy cleanrooms and biotech cleanrooms. Cleanrooms are also used by the government such as national labs, defense industries, and R&D labs at universities.
A: Most common cleanroom requirements include cleanroom classification, size, number of rooms, cleanroom flooring, cleanroom ceiling height, cleanroom chemical resistance, and cleanroom temperature and humidity.
Are you ready to build your cleanroom or need a quote for budgetary and planning? Contact American Cleanroom Systems® at (949) 589-5656 or Complete our Online RFQ Form.